The success of T cell immunotherapies in the solid tumor is impeded by challenges in part created by the secretion of metabolic byproducts from the tumor, which generate a hostile and immunosuppressive tumor microenvironment (TME) (Tan et al., 2021). Dysregulated tumor metabolism results in the accumulation of metabolites that are exported into the interstitial space in the tumor, where they act as signaling molecules promoting the immunosuppressive landscape, ultimately reducing anti-tumor responses to T cell therapies (Jiang et al., 2020; Joyce & Fearon, 2015). Tumor cells exhibit several advantages through their high metabolic plasticity, but metabolic aggression deprives surrounding immune cells of nutrients, leading to exhausted, non-functional, and suppressive phenotypes (Lim et al., 2020). Regulatory T cells (Tregs) are a central immunosuppressive component of the TME that can rewire their metabolism and obstruct anti-tumor immunity by suppressing proliferation and activation of CD8+ T cells and CD4+ helper T cells in the TME (Betts et al., 2012; McNally et al., 2011). High Treg accumulation is associated with reduced anti-tumor responses, reduced efficacy of TCR therapies and poor clinical outcomes (Onda et al., 2019; Preston et al., 2013; Tang et al., 2014). Therefore, there is an imminent clinical need for the development of more synergistic therapies that specifically target Tregs in the TME to successfully overcome the barriers of infiltration and function of antigen-specific T cells.
Accumulation of succinate, a key mitochondrial metabolite, occurs in conditions of low oxygen and increased energy demand, such the TME of solid tumors, and mutations in the gene that encoding succinate dehydrogenase ( Sdh) have been identified in a wide variety of solid tumors (Killian et al., 2013; Roh et al., 2019),resulting further an elevation of succinate levels. Accumulated succinate is secreted into the interstitial space (Garrigue et al., 2017) where it can stimulate the membrane bound succinate receptor (SUCNR1) on neighboring cells, further polarizing their immunosuppressive phenotype, and skewing macrophages to an M2 anti-inflammatory phenotype (Trauelsen et al., 2021; Wu et al., 2020). However, the effect of succinate on Tregs has not been explored. We hypothesized that succinate drives immunosuppression in the solid tumor by promoting Treg proliferation and function, leading to reduced anti-tumor responses to antigen-specific T cells.
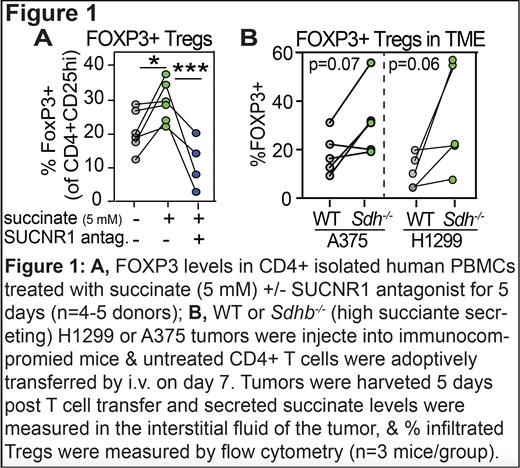
Consistent with this hypothesis, we confirmed that CD4+ T cells express the SUCNR1 and demonstrated that succinate promotes Treg numbers in CD4+ T cells isolated from healthy donor PBMCs ( Figure 1A). This effect was blocked upon administration of the SUCNR1 antagonist, NF-56-EJ40 ( Figure 1A). Deletion of the Sdhb gene, which encodes a subunit of the succinate processing enzyme, leads to intracellular accumulation of succinate. We deleted Sdhb in the non-small cell lung carcinoma cell line (H1299) and a melanoma cells line (A375) and confirmed that Sdhb -/- tumors secreted higher levels of succinate than WT. We translated these findings to a pre-clinical immunodeficient mouse model and determined that high succinate producing H1299 and A375 tumors had higher numbers of infiltrated Tregs in the TME compared with WT tumors ( Figure 1B), indicating a central role for succinate in the immunosuppressive landscape of the TME and identifying the suppression of succinate signaling as a potential therapeutic strategy to decrease immunosuppressive Tregs.
Targeting Tregs in the TME is an attractive therapeutic strategy and regulating the induction and function of Tregs has the potential to increase the efficacy of effector T cells, thus improving anti-tumor responses by removing a critical suppressive barrier. Based on these findings, we propose a novel mechanism of Treg-mediated immunosuppression driven by succinate, whereby succinate promotes a Treg-rich environment in the TME and pushes Tregs towards a Th1-suppressing phenotype. These studies identify a previously unknown molecular regulation of Tregs by extracellular succinate and provide a novel therapeutic target that has the potential to enhance the effectiveness of TCR therapy in solid tumors thereby laying a framework for translational therapeutic development and innovation, that will limit suppression of and enhance tumor-specific T cell cytotoxicity.
Disclosures
Schmitt:Lonza: Patents & Royalties: Distributions from licensed patents; Juno Therapeutics: Patents & Royalties: Distributions from licensed patents. Chapuis:Juno Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal